Featured
Boiling Point Of H2Se
Boiling Point Of H2Se. You can look them up. In the light of the above statements, choose the most appropriate answer from the options given below:
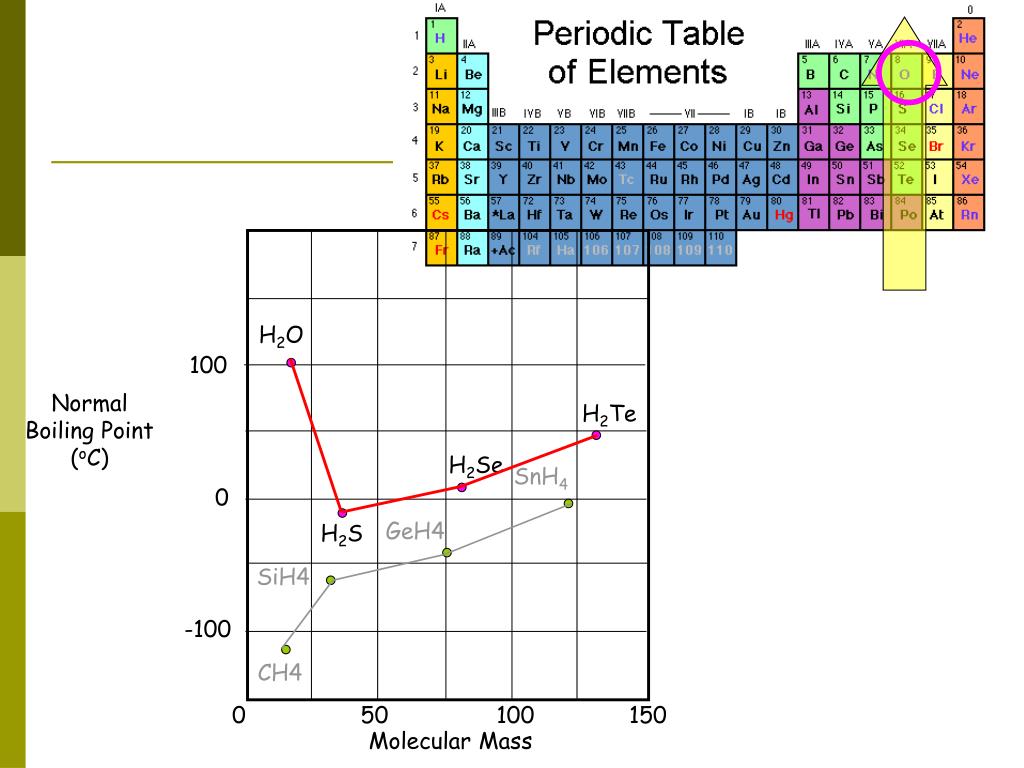
The boiling points of the following hydrides of group 16 elements increases in the order h₂o h₂s
(A) Hf (B) Hcl (C) Hi (D) Hbr
Briefly discuss why h2s has a lower boiling point than h20. When looking at what has a higher boiling point, we look at what has the strongest intermolecular forces. Briefly discuss why h2s has a lower boiling point than h2se.
However, In The Chemistry Textbook Version 6 P.484, H2Se Is Expected To Be A Stronger Acid Than H2S Because H2Se Has Weaker Bonds.
Therefore, the correct order of boiling point is: The boiling points of group 16 binary hydrides are as follows: The conjugate acid of h2se is selenonium and the conjugate base is selenide.
Boiling Points Of Simple Hydrides
I would like to see some data first. Maybe you'll get different insights into boiling point of h2se here. The boiling points of these hydrides increase with increase in molar mass.
Asked Sep 12, 2020 In Chemistry By Vijay01 (50.3K Points) Jee Main 2020;
Among h 2o,h 2s,h 2se and h 2te, the one with the highest boiling point is h 2o because of intermolecular hydrogen bonding which leads to molecular association and leads to higher energy for boiling. That means h2o is supposed to have a lower boiling point then h2s, but the opposite can be observed and thus be explained by the formation of strong hydrogen bonds. If you want an explanation, try:
It Is Soluble In Cs 2 , Phosgene.
H2s vs h2se boiling point The order of boiling point of group 16 hydrides are h 2 o > h 2 te > h 2 se > h 2 s. So that the boiling point of h 2 o is much higher than the other hydride.
Popular Posts
Which Of The Following Has The Lowest Boiling Point
- Get link
- X
- Other Apps
Comments
Post a Comment